Alkene Addition Reactions: Anti and Syn Addition
Alkene Addition Reactions: Anti and Syn Addition
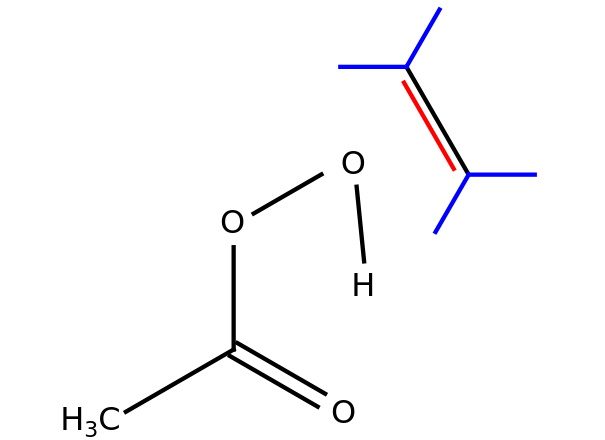
This Demonstration considers the stereospecificity of a glycol formation reaction. Both anti and syn addition are studied, with the reactions divided into two steps. Select "anti" and "step 1" to see the reaction of an alkene with peracetic acid, obtaining an epoxide[1]. In "step 2," the epoxide reacts with a base catalysis and is transformed into a glycol with the anti stereochemistry property.
Osmium tetroxide is necessary for the "syn" "step 1" case[2], so that in the reaction with alkene, a cyclic osmate ester intermediate is formed, then in "step 2" with a reducing agent, a diol is formed after the cleavage of the bond previously formed and has the syn stereochemistry property.
Details
Details
Snapshot 1: alkene double bond cleavage and epoxide is forming
Snapshot 2: base catalysis transforms the epoxide into a glycol
Snapshot 3: by using osmium tetroxide, the intermediate cyclic is obtained from the alkene
Snapshot 4: in an acid solution, with a reducing acid, the cyclic intermediate is transformed into a glycol
References
References
[1] S. Z. Lavagnino. Riassunto reattività alcheni[Video] (Jul 1, 2024) www.youtube.com/watch?v=FbPBwgWioOQ&list=PLswwssc6Q2yYB8c7fHvECjY-C3La0eDFG&index=43.
[2] LibreTexts, Chemistry, 10.7: Oxidation Reactions of Alkenes. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_I_(Liu)/10%3A_Alkenes_and_Alkynes/10.07%3A_Oxidation_Reactions_of_Alkenes.
External Links
External Links
Permanent Citation
Permanent Citation
D. Meliga, L. Lavagnino, A. Ratti, S. Z. Lavagnino, Xin Liu
"Alkene Addition Reactions: Anti and Syn Addition"
http://demonstrations.wolfram.com/AlkeneAdditionReactionsAntiAndSynAddition/
Wolfram Demonstrations Project
Published: August 27, 2024