Conversion of Ethylbenzene to Styrene in the Presence of Steam
Conversion of Ethylbenzene to Styrene in the Presence of Steam
Styrene is a monomer used in the production of many plastics. Its rate of industrial production is the fourth highest after monomers such as vinyl chloride.
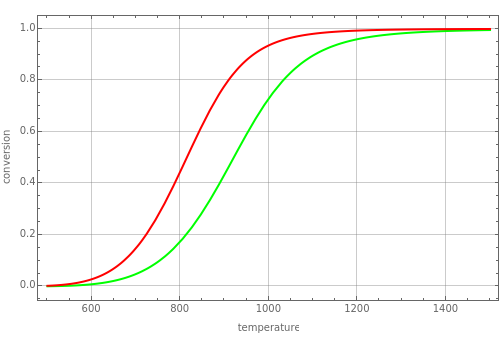
Consider the endothermic dehydrogenation of ethylbenzene (EB) to produce styrene (S) and hydrogen: . The temperature-dependent equilibrium constant is given by . This Demonstration plots the conversion versus temperature curve in red for user-set values of the steam flow rate and total pressure. In this case, steam acts as an inert component in the reacting mixture and increasing its flow rate leads to higher values of the conversion. Indeed, the green curve, which corresponds to the case where no steam is added, is always below the red curve. On the other hand, conversion decreases if total pressure (expressed in atm) is increased. In agreement with LeChatelier's rule, conversion approaches unity for very high temperatures because the reaction is endothermic.
EB⇌S+
H
2
ln()=-13.211-+4.353ln(T)-0.00329T
K
eq
13122.469
T